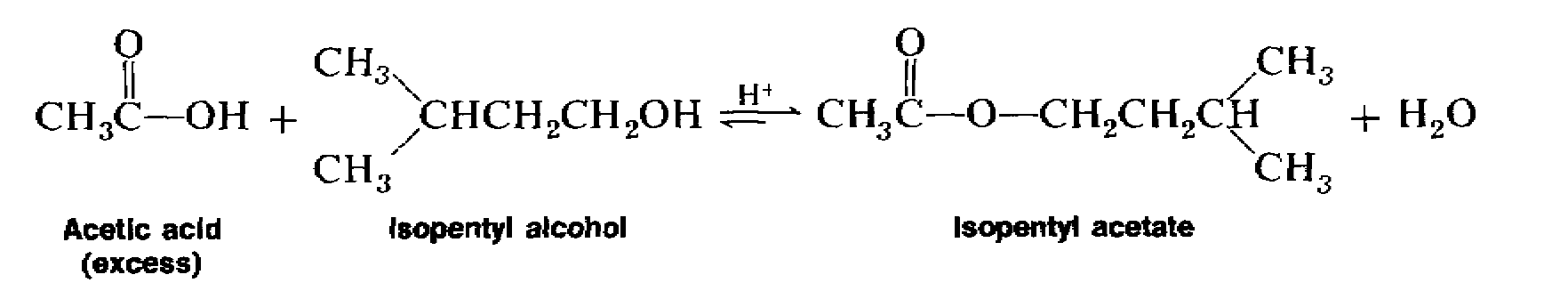
In this experiment, we will prepare an ester, according to the general
reaction below. The first compound is an organic acid, where "R"
is any group of carbons and hydrogens. The second compound is an
alcohol, where R' is also any hydrocarbon group (it can be the same as
R). When combined in the presence of acid and heated, a water molecule
is formed from the OH in each molecule, leaving a bridge over oxygen.
The resultant compound is an ester.
| R–C=O + HO–R' ®
R–C=O + HOH
| | OH O–R' |
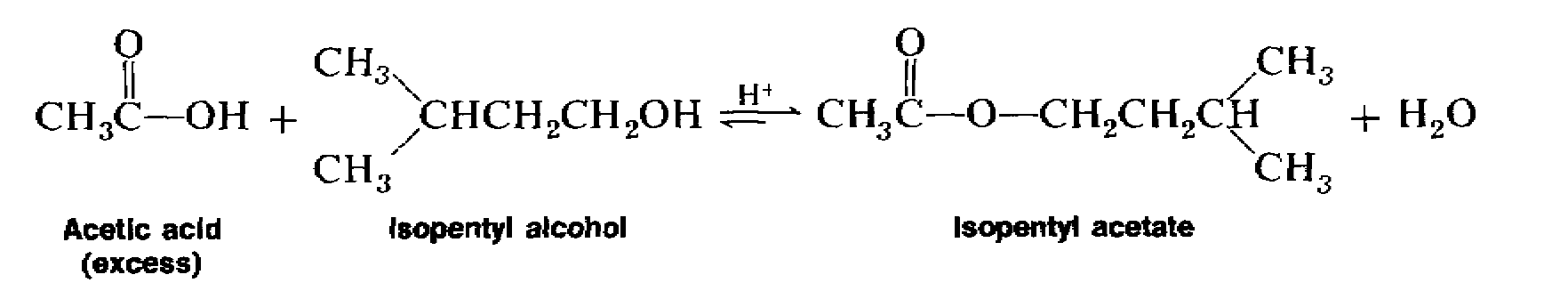
| acid | alcohol | ester | common name |
| acetic | isopentyl | isoamyl acetate | banana oil (or pear oil) |
| acetic | methyl | methyl acetate | oil of wintergreen |
| salicylic | acetic anhydride | acetyl salicylic acid | aspirin |
Isopentyl acetate (or isoamyl acetate) is often referred to as banana oil, since it has the familiar odor of this fruit. Coincidentally, this oil is also the alarm pheromone of the common honeybee.
Isopentyl acetate is prepared by the direct esterification of acetic acid with isopentyl alcohol. Since the equilibrium does not favor the formation of the ester, it must be shifted to the right, in favor of the product, by using an excess of one of the starting materials. Acetic acid is used in excess because it is less expensive than isopentyl alcohol and more easily removed from the reaction mixture.
In the isolation procedure, much of the excess acetic acid and the remaining isopentyl alcohol are removed by extraction with water. Any remaining acid is removed by extraction with aqueous sodium bicarbonate. The ester is purified by distillation.
SPECIAL INSTRUCTIONS
The reflux apparatus should be set up according to your instructor's
directions. Be careful in handling concentrated sulfuric acid. It will
cause extreme burns if spilled on the skin.
PROCEDURE
The procedure for the synthesis of Oil of Wintergreen or Aspirin is
Lab 38 of your lab textbook. The synthesis of Banana Oil is below:
Pour 15 mL (12.2 g, 0.138 mole) of isopentyl alcohol (also called isoamyl alcohol or 3-methyl-l-butanol) and 20 mL (21 g, 0.35 mole) of glacial acetic acid into a 100-mL round-bottomed flask. Carefully add 4 mL of concentrated sulfuric acid to the contents of the flask, with swirling. Add several boiling stones to the mixture.
CAUTION: Extreme care must be exercised to avoid contact with concentrated sulfuric acid. It will cause serious burns if it is spilled on the skin. If it comes in contact with the skin or clothes, it must be washed off immediately with excess water. In addition, sodium bicarbonate may be used to neutralize the acid. Clean up all spills immediately.Assemble a reflux apparatus as demonstrated. Bring the mixture to a boil with a suitable heating source, such as a heating mantle or an oil bath. Heat the mixture under reflux for 1 hour. Remove the heating source and allow the mixture to cool to room temperature.
Pour the cooled mixture into a separatory funnel and carefully add 55 mL of cold water. Rinse the reaction flask with 10 mL of cold water and pour the rinsings into the separatory funnel. Use a stirring rod to mix the materials somewhat. Stopper the separatory funnel and shake it several times as demonstrated. Separate the lower aqueous layer from the upper organic layer (density 0.87 g/mL). Discard the aqueous layer after making certain that the correct layer has been saved.
The crude ester in the organic layer contains some acetic acid, which can be removed by extraction with 5% aqueous sodium bicarbonate solution. Carefully add 25 mL of the 5% base to the organic layer contained in the separatory funnel. Swirl the separatory funnel gently until carbon dioxide gas is no longer evolved. Stopper and gently shake the funnel once or twice, and then vent the vapors. Shake the funnel until no vapors are evolved when the separatory funnel is vented. Remove the lower layer, and repeat the above extraction with 25 mL of 5% sodium bicarbonate solution. Remove the lower layer and check to see whether it is basic to litmus. If it is not basic, repeat the procedure with additional 25-mL portions of 5% base until the aqueous layer is basic.
Discard the basic washings and extract the organic layer with one 25-mL portion of water. Add 5 mL of saturated aqueous sodium chloride to aid in layer separation. Stir the mixture gently; do not shake it. Carefully separate the lower aqueous layer and discard it.
When the water has been removed, pour the ester from the top of the separatory funnel into a flask. Add about 2 g of anhydrous magnesium sulfate to dry the ester. Stopper the flask and swirl it gently. Allow the crude ester to stand until the liquid is clear. About 15 minutes will be needed for complete drying. If the solution is still cloudy after this period, decant the solution, and add to it a fresh 0.5 g quantity of drying agent.
Assemble a simple distillation apparatus as in Lab 4. Dry all glassware thoroughly before use. Carefully decant the ester into the distilling flask so that the drying agent is excluded. Add several boiling stones and distill the ester. The receiver must be cooled in an ice bath. Collect the fraction boiling between 134 and 143 °C in a dry flask. Weigh the product and calculate the percentage yield. To the best of your ability, determine the density.
Results
boiling point during final distillation:
mass of ester produced:
predicted yield (based on amounts of each reactant):
percentage yield:
density:
"official" BP:
"official" density:
Health Hazards (consult the Merck Index)