 group
attached. Notice that these molecules have the -OH group in them, but
they are alcohols because of the additional double-bonded oxygen. This
group is usually written as -COOH when typing.
group
attached. Notice that these molecules have the -OH group in them, but
they are alcohols because of the additional double-bonded oxygen. This
group is usually written as -COOH when typing.MHS Chemistry
Common Organic Acids
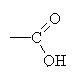
In organic chemistry, acids are molecules made mostly of carbon and hydrogen,
with a  group
attached. Notice that these molecules have the -OH group in them, but
they are alcohols because of the additional double-bonded oxygen. This
group is usually written as -COOH when typing.
group
attached. Notice that these molecules have the -OH group in them, but
they are alcohols because of the additional double-bonded oxygen. This
group is usually written as -COOH when typing.
The acids listed below are the acids we are likely to have available for the Synthesis of Esters lab. In each diagram, every bend or end of a line is a carbon atom, unless otherwise shown. Every carbon atom makes four bonds, and every bond not shown is a bond to a hydrogen atom. Also, each image is also a link to its originating page.
| CH3(CH2)16COOH stearic acid |
|
| CH3(CH2)10COOH lauric acid |
|